1860 – Germany
Each chemical element, when heated to incandescence, produces its own characteristic lines in the spectrum of light
For example, sodium produces two bright yellow lines.
Bunsen developed the Bunsen burner in 1855.
In the flame test the Bunsen burner’s non-luminous flame does not interfere with the coloured flame given off by the sample.
Kirchhoff was a professor of physics at Heidelberg. Bunsen and Kirchhoff together developed the first spectroscope, a device used to produce and observe a spectrum. They used their spectroscope to discover two new elements, caesium (1860) and rubidium (1861).
In 1860 Kirchhoff made the discovery that when heated to incandescence, each element produces its own characteristic lines in the spectrum.
This means that each element emits light of a certain wavelength – sodium’s spectrum has two yellow lines (wavelengths about 588 and 589 nanometres). The Sun’s spectrum contains a number of dark lines, some of which correspond to these wavelengths.
The Swedish scientist ANDERS ANGSTROM had, four years earlier, found that a gas always absorbs light at the same wavelength that it emits light. If the gas is hotter than the light source, then more light is emitted by the gas than absorbed, creating a bright line in the spectrum of the light source. If the gas is cooler than the light source the opposite happens; more light is absorbed by the gas than is emitted, creating a dark line.
The dark solar D lines told Kirchhoff that sodium is present in the relatively cool outer atmosphere of the Sun. This could be tested in the laboratory by burning a piece of chalk in a hot oxygen-hydrogen torch. The intensely bright limelight that is produced may be passed through a cooler sodium flame and the light emerging examined through a spectroscope. Crossing the spectrum of the artificial light occur black lines at the same wavelength that a sodium flame emits light. This solved the mystery of the FRAUNHOFER LINES.
Scientists now had a means to determine the presence of elements in stars. By comparing the dark lines in the spectra of light from the stars with the bright lines produced by substances in the laboratory, Kirchhoff had been able to identify the elements that made up a celestial body millions of miles away in space.
In England the astronomer William Huggins recorded the spectra of hundreds of stars and showed the unmistakable fingerprints of familiar elements that are found on the Earth’s surface. The stars are made of exactly the same kind of atoms as the Earth.
In 1868 Norman Lockyer described a spectral line in the yellow region very close to the wavelength of the two ‘D’ spectral lines of sodium. After repeated attempts to discover a substance that produced the same line on Earth, it appeared that the line did not correspond to any hitherto known element. Lockyer gave the element the name ‘helium’, the gas later to be found associated with radioactive decay in ores containing uranium.
Helium had not previously been found on Earth because it is both inert and lighter than air, ironic because after hydrogen, helium is the second most common element in the universe.
In 1904 RUTHERFORD would declare that the presence of helium in the Sun was evidence that sunlight was a product of radioactive processes. The absence of any FRAUNHOFER lines in sunlight that corresponded to radium dealt a blow to this hypothesis. Was there another way of releasing atomic energy than radioactivity?
Related sites
- spectroscopy (www.dmoz.org)
- BunsenBurner download .pdf
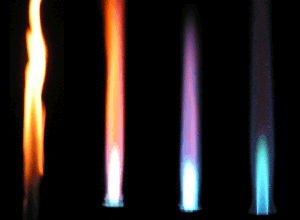



 TIMELINE
TIMELINE